Take Part in a Movement
Are you receiving various treatments for your chronic knee pain and not seeing long-term results? You may be eligible to take part in Curonix’s Freedom-1 Study.
Peripheral Nerve Stimulation (PNS) has been gaining popularity in recent years as it is effective, safe and less invasive than surgery for the treatment of chronic pain.1 The Freedom-1 Research Study focuses on the use of our Freedom® Peripheral Nerve Stimulator (PNS) System to possibly relieve chronic pain and improve overall quality of life.
Peripheral Nerve Stimulation (PNS) has been gaining popularity in recent years as it is effective, safe and less invasive than surgery for the treatment of chronic pain.1 The Freedom-1 Research Study focuses on the use of our Freedom® Peripheral Nerve Stimulator (PNS) System to possibly relieve chronic pain and improve overall quality of life.
Freedom Stimulators are:
• Non-Drug, Non-Opioid
• Minimally-Invasive
• Typically, an Outpatient Procedure
• Full-Body Conditional at 1.5T and 3T MRI Scans2

Our Solution for Chronic Knee Pain Relief
We offer the Freedom PNS System with an implantable stimulator that may help relieve pain by sending electrical stimulation to a specific nerve near your knee, blocking the pain signals from reaching your brain. The system is designed to seamlessly integrate into your daily life and help you take control of your chronic pain.
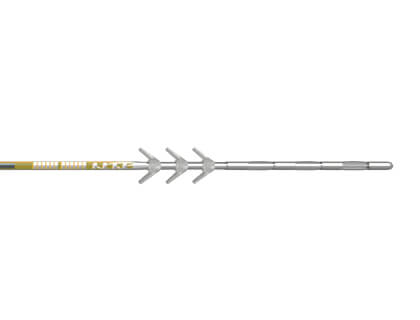
Implanted
Stimulator
The implantable stimulator is intended to block pain signals to the brain, thereby helping to relieve chronic pain.

Transmitter Assembly
(Transmitter + Antenna)
The external transmitter houses the battery and provides power to the antenna, which sends a signal through the skin to the implanted stimulator to help relieve pain.

PNS Extremity
Wearable
Our wearables hold the transmitter assembly. They are designed for comfort and should be worn over a thin layer of clothing.
What to Expect in The Freedom-1 Study
During your journey with the Freedom PNS System, your healthcare team will assess your pain levels and physical function through a series of scheduled appointments.
Here is an overview of what your journey might look like:
Here is an overview of what your journey might look like:
Ensuring you are the right candidate
Your healthcare professional will meet with you to ensure the Freedom PNS System is right for you and if you are eligible to be a part of the study.
Confirming Your Pain Location
You will undergo a nerve block injection to ensure that the exact nerve that is causing your chronic pain is being treated. This may provide immediate, temporary relief.
Trial Period
Determine the benefits of the Freedom PNS System firsthand during a trial period—helping assess the therapy’s effectiveness for both your physical and psychological well-being. During this time, in collaboration with your healthcare team, you will decide if this device is right for you.
Permanent Implant
Upon successful completion of the trial period, you will work together with your healthcare provider to discuss next steps. This includes coordination of your permanent implant procedure and how to use the Freedom PNS System, ensuring long-term support and care for your pain relief needs.
Please Note: If you choose to enroll, you will be a part of a double-blinded study. A double blinded study means you and your physician will not know which group you will be in until approximately 30 days after implantation. This helps eliminate bias and ensures reliable results.
Please Note: If you choose to enroll, you will be a part of a double-blinded study. A double blinded study means you and your physician will not know which group you will be in until approximately 30 days after implantation. This helps eliminate bias and ensures reliable results.
Follow Up Appointments
Appointments with your healthcare professional will take place in-person at 1 week, 1 month, 3 months and 6 months after your permanent procedure. During these times, your healthcare provider will ask you a series of questions regarding your pain and functionality.
After the first 6 months, you will participate in 3 remote follow-up appointments to complete the study participation of 2 years.
After the first 6 months, you will participate in 3 remote follow-up appointments to complete the study participation of 2 years.
Am I a Candidate?
Complete the assessment to see if you are a candidate to take part in a clinical study.
"*" indicates required fields

