Our Technology
Freedom SCS System
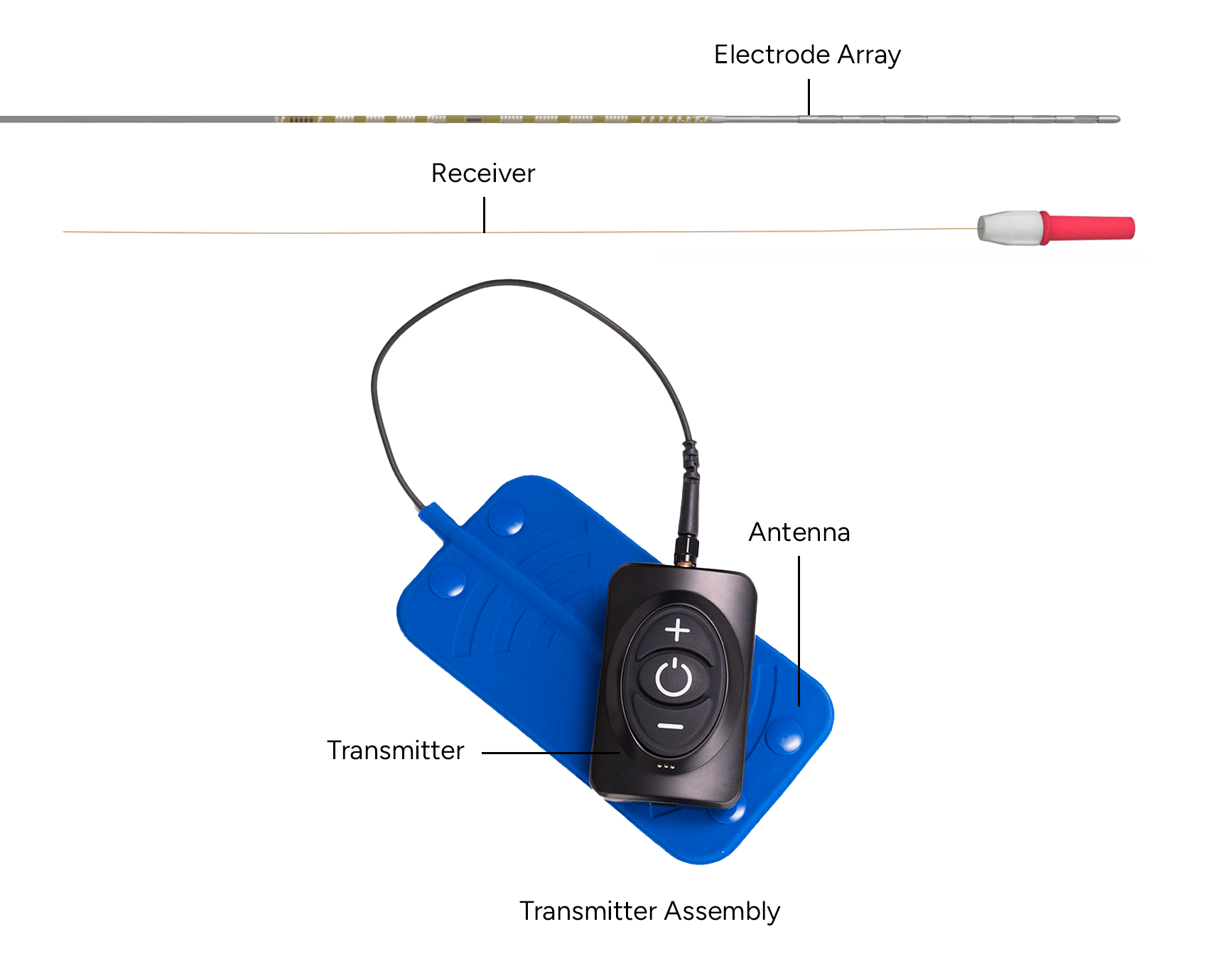
The Freedom SCS System uses High Frequency-Electromagnetic Coupling (HF-EMC) technology to power the implanted neurostimulator. Each stimulator comprises of an electrode array(s) with eight contacts, and the electrode array(s) are connected to a separate implanted receiver(s). A small, external rechargeable transmitter supplies the power and data to the implanted neurostimulator through the skin. The device uses pulsed electric current to create an electrical field that acts on nerves to inhibit the transmission of pain signals to the brain.

SCS Wearable
The HF-EMC Technology powers the device through clothing*, offering patients a comfortable and discreet option for holding their external transmitter during daily activity.
*Internal testing on file with Curonix.

Patient Selection
Commonly, patients will present with the following conditions and/or medical history:
- Post-Surgical Chronic Pain
- Successful Epidural Injections
- Successful RFAs
- Comorbidities
- Failed Back Surgery Syndrome
- High MRI Burden
- Non-surgical Candidacy
If the Freedom SCS System is right for your patient, they will first undergo a trial period to assess the effectiveness of chronic pain management before transitioning to a permanent implant.


