Our Technology
The Freedom® Peripheral Nerve Stimulator (PNS) System is indicated for pain management in adults with severe, intractable chronic pain of peripheral nerve origin, as the sole mitigating agent, or as an adjunct to other modalities of therapy used in a multidisciplinary approach.
Freedom PNS System
The system uses High Frequency-Electromagnetic Coupling (HF-EMC) technology to power the implanted neurostimulator.
- Each stimulator comprises of an electrode array(s) with four or eight contacts, and the electrode array(s) is connected to a separate implanted receiver(s).
- A small, external rechargeable transmitter supplies the power and data to the implanted neurostimulator through the skin.
- The device uses pulsed electric current to create an electrical field that acts on nerves to inhibit the transmission of pain signals to the brain.
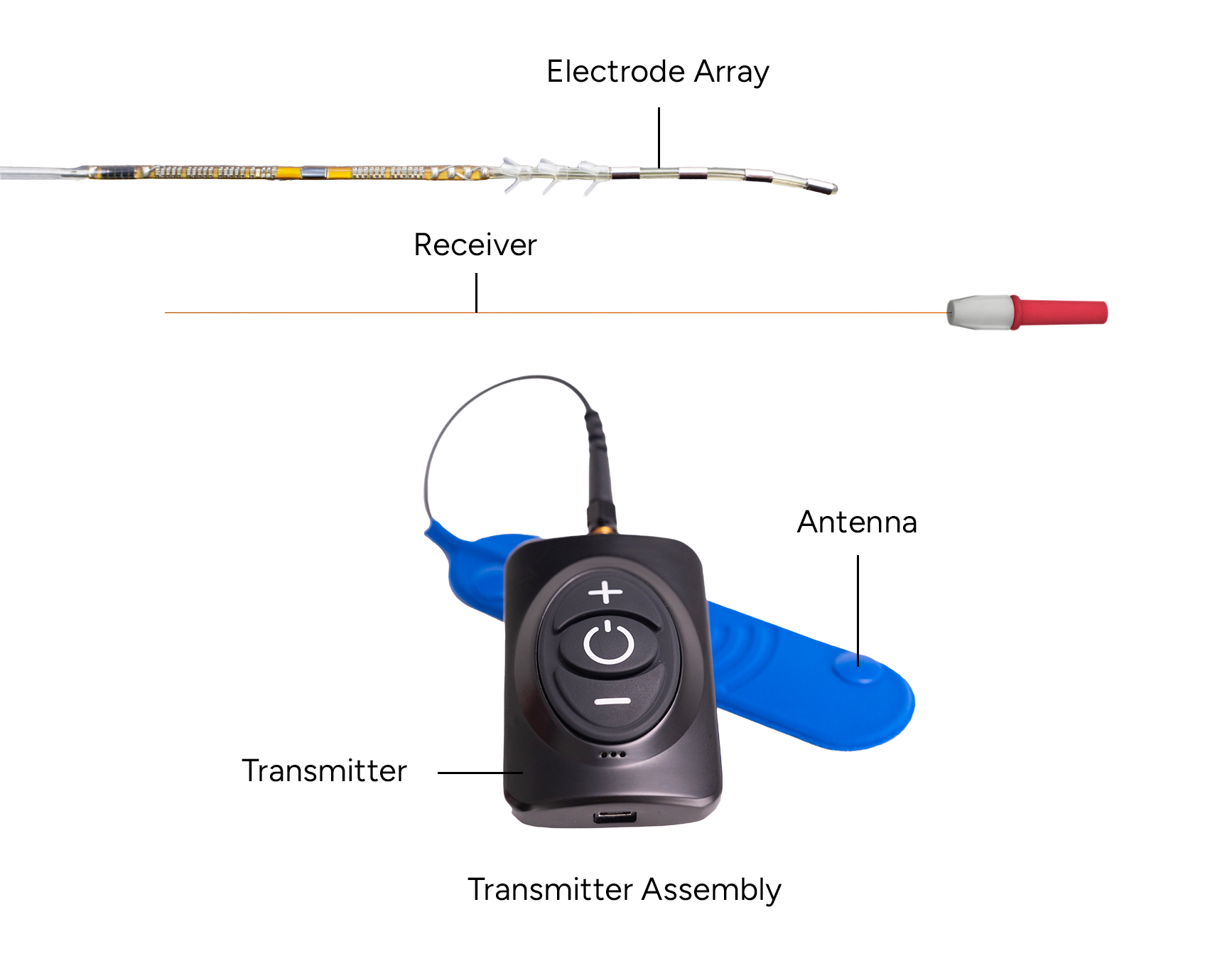
NervPulse™ Therapy
- Therapy programs for 26* various peripheral nerves.
- Evidence-based data from 20,000 Freedom PNS procedures and 13 publications with 450+ study patients and growing.
- Leveraging battery testing, quality and device data to optimize device performance.
- Streamlines the programming process for patients and enhances workflow efficiency, leading to better patient outcomes.
*Internal data on file with Curonix.

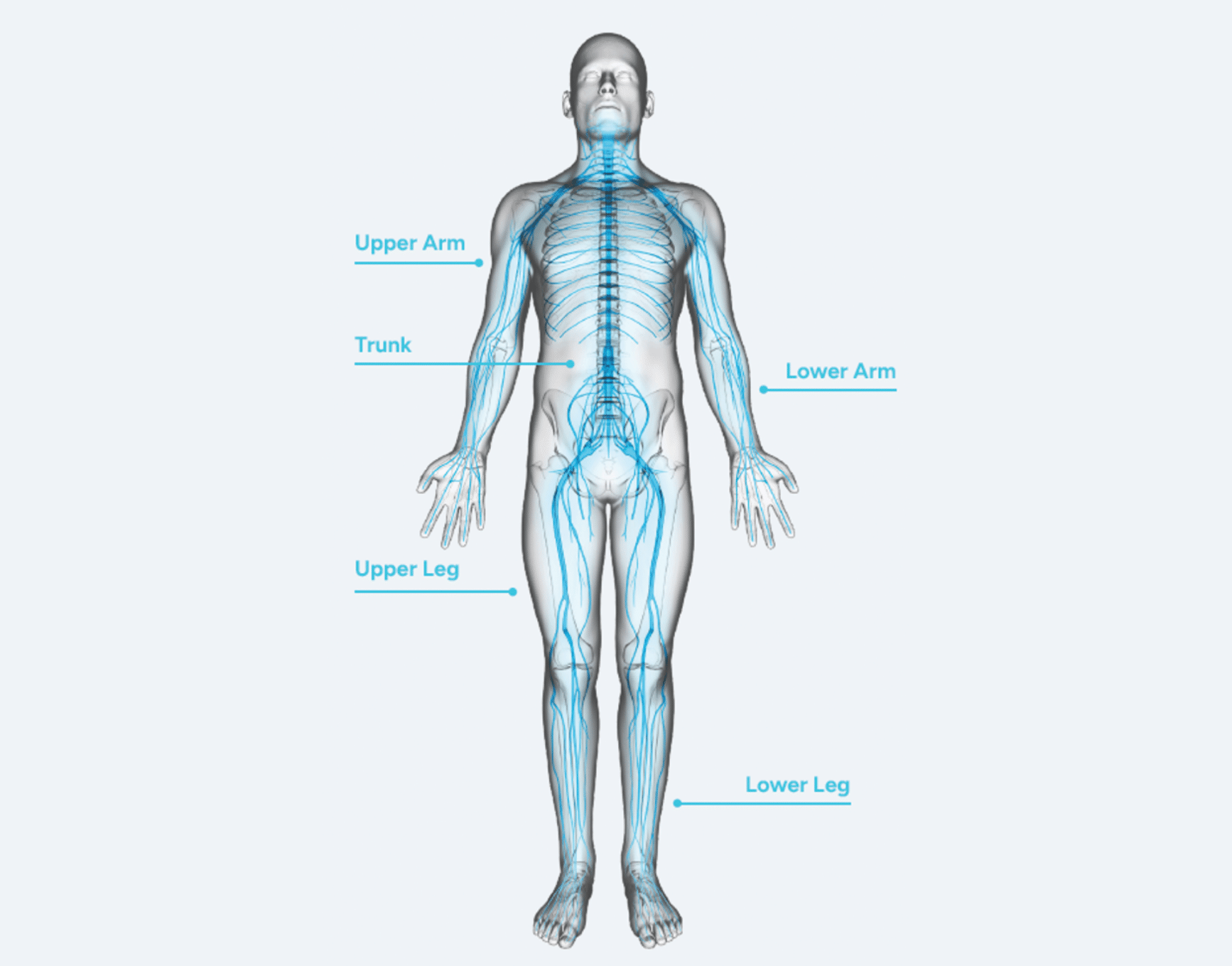
PNS Wearables
The wearables are purposefully designed to work with the Freedom PNS System for any extremity or trunk nerve approach.
The HF-EMC Technology powers the device through clothing*, offering patients a comfortable and discreet option for holding their external transmitter during daily activity.
*Internal testing on file with Curonix.


Patient Selection
Commonly, patients will present with the following conditions and/or medical history:
- Post-Surgical Chronic Pain
- Successful Nerve Block
- Mononeuropathies
- Successful RFAs
- High MRI Burden
- Failed SCS
- Blood Thinner Dependent
- Comorbidities
If the Freedom PNS System is right for your patient, they will first undergo a trial period to assess the effectiveness of chronic pain management before transitioning to a permanent implant.

Clinical Evidence
Decrease in Medication Usage
Patients reduced medication usage by 47% at 24 months post-implant. 1
Reduced Pain
Patients reported more than a 70% improvement in pain at all follow-ups. 1
Foot and Ankle patients experienced a 65% reduction in pain scores at 12 months post-implant. 2
Foot and Ankle patients experienced a 65% reduction in pain scores at 12 months post-implant. 2
Improvement in Quality of Life
Foot and Ankle patients experienced an improvement in quality of life 12 months following PNS implantation. 2
Frequently Asked Questions
Can patients receive an MRI with Freedom PNS?
Does the patient always keep the device on to receive therapy?
How will your patient know where to place the wearable and external transmitter assembly?
Provider Resources
Get access to additional educational content, including videos and more.
Learn More
References:
1
Abd-Elsayed, Alaa, and Robert Moghim. “Efficacy of Peripheral Nerve Stimulation with a High Frequency Electromagnetic Coupled (HF-EMC) Powered Implanted Receiver in Treating Different Pain Targets/Neuralgias.” Journal of Pain Research (2023): 589-596.
2
Pollina, Ryan, Gabriela Betanzons, and Alaa Abd-Elsayed. “Peripheral Nerve Stimulation With a High-Frequency Electromagnetic Coupled Powered Implanted Receiver at the Posterior Tibial Nerve for the Treatment of Chronic Pain in the Foot.” Neuromodulation: Technology at the Neural Interface (2023).

