Market Leader in PNS & MRI
An MRI scan can be a crucial part in planning chronic pain treatment. Treat your patients with Freedom PNS knowing they can receive both a full-body 1.5T and 3T scan in the future, when medically necessary.
Important Safety Information
Magnetic resonance imaging (MRI) may be safely performed under certain conditions on a patient with a Freedom Stimulator System (SCS/PNS).
The Transmitter Assembly unit (i.e., the external transmitter and antenna components of this neuromodulation system) MUST NOT be present in the MRI system room at ANY TIME. Failure to adhere to the specific requirements described in this manual can result in tissue damage, severe injury, or death.
A patient with the permanent Freedom implant specified below can be safely scanned in an MR system meeting the conditions defined in the table below, per the location of the device implant.
For more safety and patient information on MRI, please contact (800) 965-5134 or see the MRI Instructions for Use at the button below.

 The following components are MR Unsafe: FR4A Trial Lead, FR8A Trial Lead, External Transmitter Assembly, Programmer, USB Battery Charger, Introducer, Guidewire, Steering Stylet For more information on preparation for an MRI, undergoing an MRI and post-MRI, please see the IFU.
The following components are MR Unsafe: FR4A Trial Lead, FR8A Trial Lead, External Transmitter Assembly, Programmer, USB Battery Charger, Introducer, Guidewire, Steering Stylet For more information on preparation for an MRI, undergoing an MRI and post-MRI, please see the IFU.Use the toggle button for more information on MR guidance based on the MRI scanner
Permanent Freedom Peripheral Nerve Stimulator (PNS) System STQ4 and FR4A
Includes the following permanent implants:
STQ4-RCV-A0, STQ4-RCV-B0, STQ4-SPR-B0, FR4A-RCV-A0, FR4A-RCV-B0, FR4A-SPR-B0


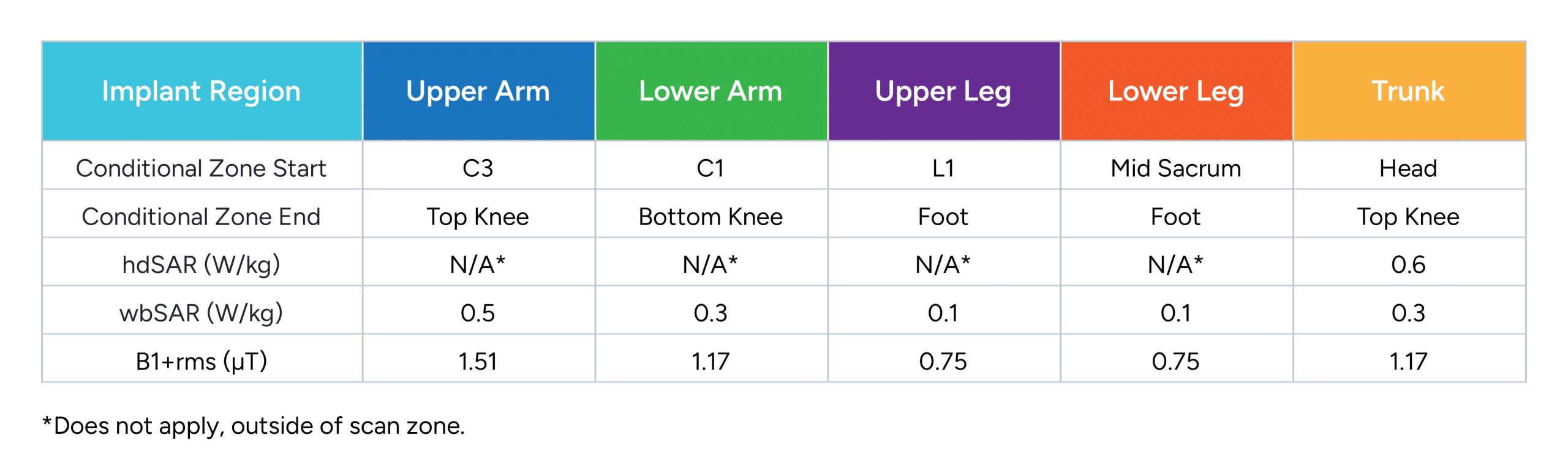
Permanent Freedom Peripheral Nerve Stimulator (PNS) System FR8A
Includes the following permanent implants:
FR8A-RCV-A0, FR8A-RCV-B0, FR8A-SPR-B0


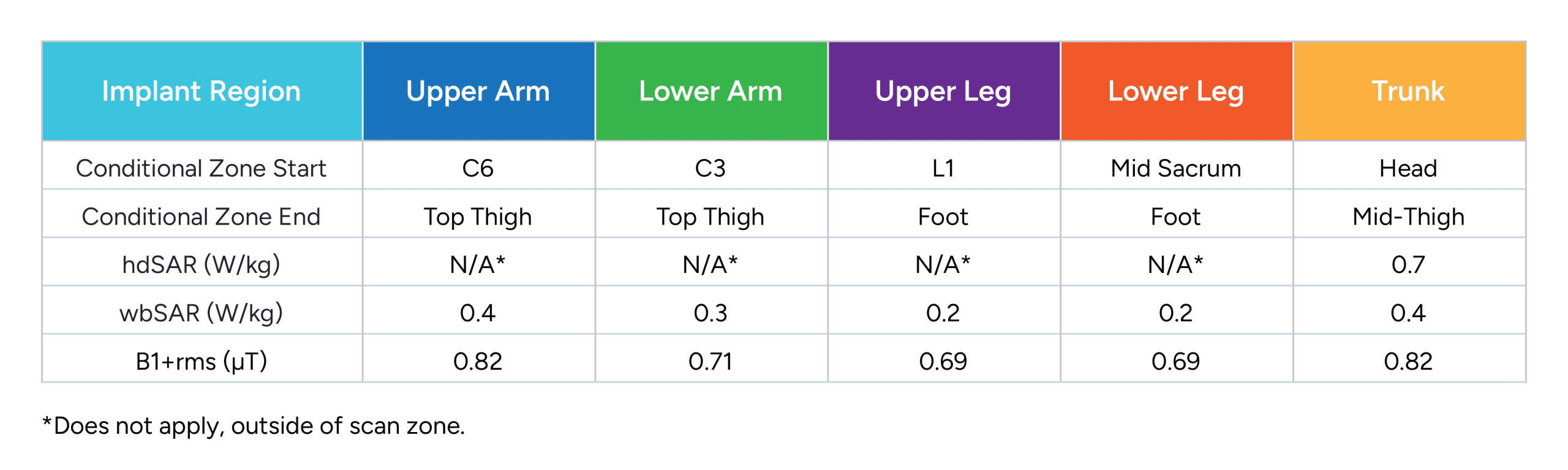
Permanent Freedom Spinal Cord Stimulator (SCS) System FR8A
Includes the following permanent implants:
FR8A-RCV-A0, FR8A-RCV-B0, FR8A-SPR-B0



Permanent Freedom Peripheral Nerve Stimulator (PNS) System STQ4 and FR4A
Includes the following permanent implants:
STQ4-RCV-A0, STQ4-RCV-B0, STQ4-SPR-B0, FR4A-RCV-A0, FR4A-RCV-B0, FR4A-SPR-B0


Permanent Freedom Spinal Cord Stimulator (SCS) System FR8A
Includes the following permanent implants:
FR8A-RCV-A0, FR8A-RCV-B0, FR8A-SPR-B0




